Kite turns out to be a concerning investment for Gilead When Gilead acquired Kite for $12 billion (!), it was the talk of the industry for many months. It was a sign that Gilead was investing big in CAR-T...
Kite turns out to be a concerning investment for Gilead
When Gilead acquired Kite for $12 billion (!), it was the talk of the industry for many months. It was a sign that Gilead was investing big in CAR-T cell therapy. Things have soured since, with Gilead cutting forecasts for drugs in the Kite pipeline. This was discussed in an earlier post.
This week, Gilead further cut the accounting value for research assets by $800 million, thus signaling that it is placing lower expectations on Kite’s pipeline. So far, only Yescarta from Kite has been able to generate Revenue for Gilead, but its revenues are far from making it a worthwhile investment. With the rest of the pipeline looking less promising, the Kite deal may not have been a good idea for Gilead after all.
Regeneron sets sights on coronavirus vaccine
In view of the World Health Organization declaring the Coronavirus outbreak a global public health emergency, more companies are entering the fray to develop a vaccine. Last week I wrote about J&J launching its vaccine development efforts. This week, Regeneron announced that it will be working in tandem with the US government to develop its own vaccines.
Regeneron definitely has a smaller global footprint than J&J w.r.t. distribution and delivery of the vaccine. However, with the US government on its side, it may very well be able to have the resources necessary to overcome that hump. At this point, it is purely a race to see who can come with a reliable vaccine in the shortest amount of time
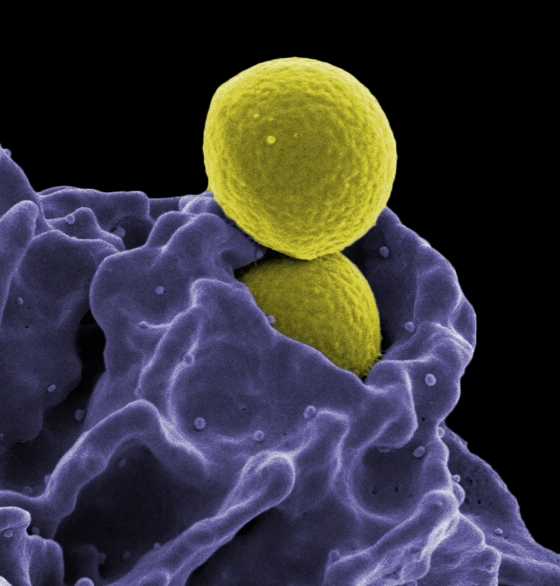
Move over, CAR-T cells. CAR-NK cells next?
A small study led by MD Anderson in collaboration with Takeda has demonstrated the efficacy of CAR-NK cells in CD19+ cancers (non-Hodgkin’s lymphoma or chronic lymphocytic leukemia). The study showed similar efficacy to conventional CAR-T cell therapy that also targets CD19, but provided a way to do away with the negative effects of CAR-T cell therapy.
There are a number of advantages that CAR-NK therapy provides:
NK cells are the first line of defense that can later induce antigen-specific T cell responses to the tumor. Thus it leads to two waves of ‘hits’ at the tumor, thus amplifying the overall anti-tumor immune response
The authors were able to use cord blood instead of patient’s own blood. This avoids the long process of genetically modifying the patients T cells for CAR-T cell therapy, making sure patients receive treatment in a timely manner
The results from this study need to be interpreted with care, though. This was an extremely small study with only 11 patients. In addition, the patients need to be monitored for longer periods of time to look for adverse effects as well as prolonged efficacy.







